The field of major histocompatibility complexes (MHCs, see box) has long been a bit of a neglected child. Sjaak Neefjes and Huib Ovaa had an interest in the field though, both working at the NKI in Amsterdam about a decade ago. With several collaborations between the groups already ongoing, Ferenc Scheeren, group leader at the department of Dermatology at LUMC, also became involved in the work on MHC-I. He says: "I had an interest in technology, just like Huib. We had a good relationship at work and in private and we received a large grant together. The work on MHC was a small part of Huib's research, but it got a lot of love from him."
A conserved target
As part of this interest in MHCs, Scheeren and Ovaa started a collaboration with Paula Ruibal and Simone Joosten at the department of Infectious diseases at LUMC, aimed at vaccine development against infection with Mycobacterium tuberculosis (Mtb), including technology development such as thermal peptide exchange.
After a challenging few years, in which the labs were not functioning as normal because of the COVID-19 pandemic and on top of that the passing of Huib, Neefjes asked Scheeren to take over the MHC research from the Ovaa-lab, including the project on thermal peptide exchange. Added to the group was Ian Derksen, who had been developing the method in the Ovaa-lab under Jolien Luimstra's supervision. As an expert in the technique, he continued his work under supervision of Scheeren and Ruibal.
Instead of the most studied classical MHC-I allele HLA-A*02:01, Ruibal's group studied peptides that bind to the non-classical HLA-E to investigate immune responses against an Mtb infection. Ruibal explains: "While HLA-A*02:01 is most abundant in the Caucasian population, HLA-A molecules are genetically highly diverse. HLA-E is much more conserved between people. Most people express the same alleles; we know two alleles which differ by only one mutation. That means if you find an antigen or peptide that you, for instance, want to base a vaccine on, we expect that most of the population has the same response and thus that the vaccine will be effective for most people."
Serendipity
Studying a different MHC-I allele meant that Derksen had to re-design his peptide exchange method to work for HLA-E. He explains: "First, we needed to find a new template peptide. Finding it was real serendipity. I received a peptide which had a synthetic error in it: one of the amino acids was accidentally added to the original peptide. But this one turned out to be the best."
Then he had to adapt the method for thermal exchange. "This was difficult. Everything with HLA-E is more difficult. We managed to exchange 80% of the peptides in the best case, whereas for HLA-A*02:01 you can exchange more than 95%."
Derksen found a smart workaround for this: instead of first making HLA-E tetramers and then performing the thermal peptide exchange, he performed the thermal exchange using HLA-E monomers and then made the tetramers. In this way the tetramers, which are used for the immunoassays, are loaded with the peptide of interest at similar rates to those of HLA-A*02:01.
Easy-to-use platform
The efforts have led to a greater understanding about producing MHC-I-peptide complexes, which the researchers want to share with the world. Sjaak Neefjes, Huib Ovaa together with Malgorzata Garstka and Jolien Luimstra have filed a patent for the technology. Importantly, using this technology, MHC-I-tetramers with a given peptide can very easily be produced. Clinical researchers with no specific expertise can then use these in immunoassays, such as Ruibal is doing. "It is suitable as a high-throughput technology, which we can use to screen many peptides and select the best to further investigate immune responses that could be involved in a potential vaccine."
Scheeren believes the technology can help to design better vaccines. "To fully engineer a vaccine, you need to know the dominant T-cell epitopes; the peptides that are presented by the MHC and recognised by T-cells. This exchange technology makes it possible to go there. That is why we developed the platform."
Scheeren meanwhile is looking ahead to expand the technology. "I look forward to completing the projects Huib started and then to stand on his shoulders and move on. One thing we are working on is the development of a quality control tool for thermal peptide exchange. Additionally, I think it is important to expand the technology to include more diversity. HLA-A*02:01 is a predominantly Caucasian phenotype. I want to include a wide range of alleles within the MHC field to identify peptide antigens in people with different geographic backgrounds."
How it works
Major Histocompatibility Complex
Major histocompatibility complexes, or MHCs, mediate an ingenious part of the adaptive immune system. Class I MHCs, expressed on the surface of nucleated cells, have one purpose: to present fragments (peptides) from proteins synthesised within the cell to cytotoxic (killer) T-cells on the cell surface. There, killer T-cells scan these peptides by binding to the peptide-MHC-complex. If the peptide antigens are foreign, for example originating from a virus or tumour, the infected or mutated cell is destroyed and the antigen-specific T-cells proliferate in search of other affected cells. The ability of adaptive immune cells to distinguish between self and foreign proteins is essential. If the process fails, this can lead to autoimmunity.
Monitoring the presence of antigen-specific T-cells can provide information about, for example, a patient's immune system and former infections. T-cell specificities can be investigated using MHC-I tetramers loaded with a specific antigenic peptide, for example originating from a melanoma cell from a patient. Therapies can then include isolation and expansion of these T-cells and re-introducing them to boost the immune reaction to affected cells.
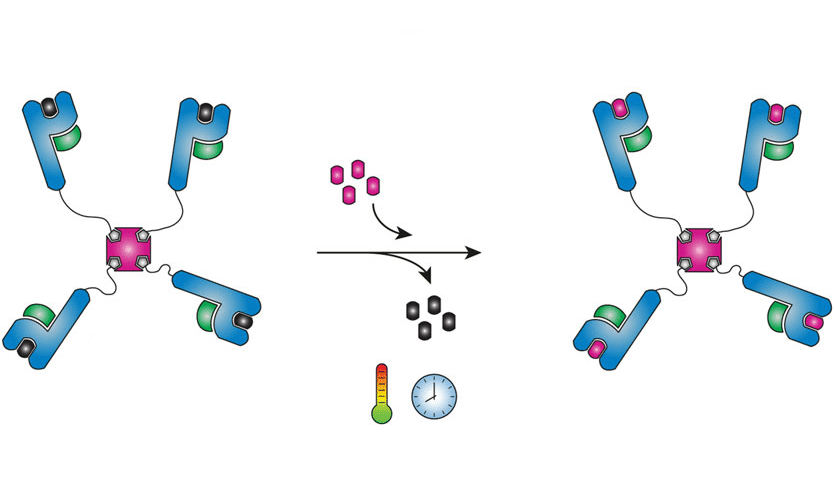
But producing and purifying MHC-I-peptide complexes is a tedious process that takes days per peptide of interest. An easier way is to produce one batch of tetramers loaded with a so-called template peptide, which can be exchanged for a peptide of choice. An example of an established method employs a photocleavable peptide that can be exchanged by exposure to UV. This technology has some drawbacks, however, including the sensitivity of biomolecules to UV radiation. In the lab of Sjaak Neefjes, the idea of using differences in thermal stability to facilitate exchange was born.
For this thermal peptide exchange, MHC-I tetramers are complexed with a template peptide, selected for its ability to form a complex that is stable at low temperatures (preferably below 10°C), but dissociates at higher temperatures (ideally room temperature). The complexes of conditional MHC-I tetramers can be exchanged for any peptide of interest that binds more stably at higher temperatures than the template peptide. In short, template-MHC-I-complexes are mixed with a solution containing a new peptide of interest and heated to room temperature, thus exchanging the peptides and forming new complexes in a very simple, time-effective manner. This facilitates the use of high-throughput methods for example to quickly scan many MHC-I-peptide complexes for T-cell recognition.
In a close collaboration, former PhD student Jolien Luimstra and student Ian Derksen, both working in the Ovaa-lab at that moment, carried out the research. NKI and LUMC have filed a patent application for the temperature-mediated peptide exchange technology.
This article was published in ICI Bulletin 15, June 2023.