Correct folding and maintenance of proteins through an extensive quality control (QC) system in cells is essential to our health. Failures can lead to disease, including Alzheimer, Parkinson, metabolic diseases, and cystic fibrosis.
The cellular QC system is managed by several dozens of chaperones, complemented by a wealth of other factors including those catalyzing post-translational modifications and degradation. Together this interactome determines the path of 20,000 proteins through the cell, from cradle to grave. Despite extensive research, there are many gaps in our knowledge, preventing the development of effective therapies.
The coordinator and one of the principal investigators (PIs) of FLOW is Ineke Braakman, professor of cellular protein chemistry at Utrecht University. The consortium consists of an extensive team of 13 other PIs from universities and UMCs all over the Netherlands. "The call that we got the grant came as a big and pleasant surprise. We are elated as we really believe in our program," said Ineke Braakman.
Mapping protein fate
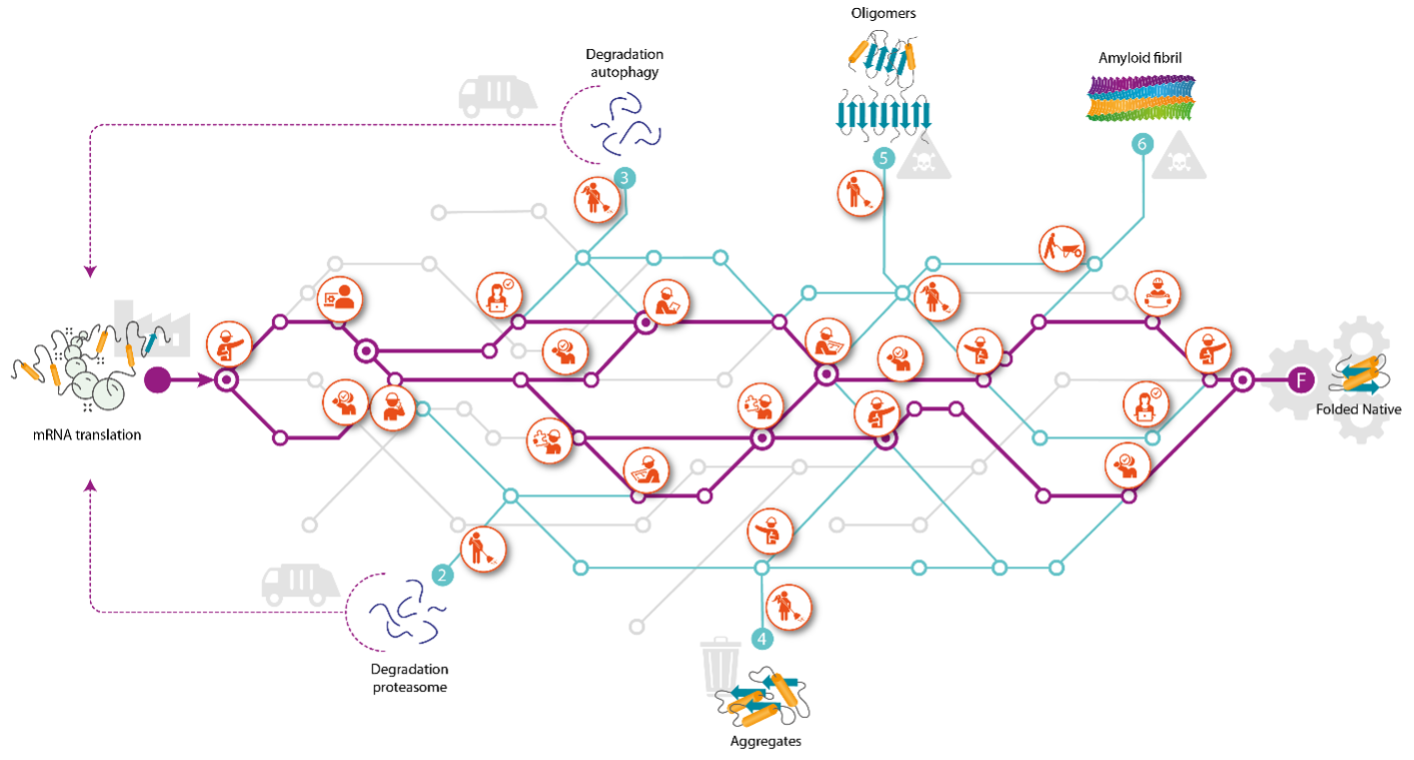
The FLOW project proposal compares the path of proteins through the cell to a subway network. Already during its synthesis, each protein travels from station to station. At each station, a molecular network including chaperones conduct a QC triage to not only help the traveling protein but also decide where the protein will go next. Many of the individual stations and tracks are known, but remarkably, there is no protein for which the entire lifecycle has been mapped.
The aim of the program is to fill in the blanks and understand the entire interactome for two model proteins: CFTR and alpha-synuclein (aSyn). CFTR is defective in the loss-of-function folding disease cystic fibrosis. Misfolding of aSyn leads to Parkinson, a gain-of-toxicity disease. Together, CFTR and aSyn face every possible fate a protein can experience in cells, making them the optimal proteins to study in FLOW.
Braakman, an expert on membrane-protein folding and of CFTR folding and fate in particular, explains: "Many publications feature CFTR but we still do not fully understand how CFTR navigates the cell and how chaperones and other cellular factors influence CFTR. Even for simple proteins there is no map of their fate from cradle to grave."
The reason is that researchers have been using different approaches, says Braakman: "Each protein and each cellular process requires a dedicated assay, which is not necessarily suitable for other proteins or processes. In our biochemical folding studies in cells, large proteins are optimal, because they undergo many modifications and conformational changes. But for in-vitro research on protein refolding, smaller proteins are easier and more informative to work with. Every assay has its own ideal proteins. Within FLOW, we aim to develop the approaches to study all life phases for these two proteins, with the intent to expand to many more. We expect that the approaches we will develop will enable us to map the life cycle of other proteins much faster."
From discovery to control
Three work packages constitute the FLOW program: discover, rebuild, and control. The first one aims to 'establish all possible fates for the two model proteins, produce an inventory of their interaction partners with spatio-temporal resolution, and establish the effects of client and QC-factor variation on the cell.'
Next, 'building is understanding' as chemists say: the team plans to recreate the triage processes in the cell that decide on the fate of the two proteins. Braakman explains: "The idea is that when we know how to reconstitute the process, we will also know how to influence it. If, for example, we understand how aSyn misfolds in Parkinson, recreating the misfolding process may teach us how to prevent it."
The last workpackage aims to reach full control over the fate of these two proteins in cells, and expand to tissues and organisms as well as to additional proteins. The ultimate goal of FLOW is to control cell health, and through this, human health.
First results
Many disciplines will collaborate in FLOW, including biochemists, biophysicists, cell biologists, and computer scientists. In a joint hiring effort this summer, the majority of 19 vacancies was filled with young researchers. The program has its first kick-off meeting in November, which will include speed dates between new researchers of all disciplines. Braakman: "The recruitment symposium already generated a great culture of togetherness among the PIs and the new hires. This collaboration is so important for our research. The questions we aim to answer in FLOW are crucial for cell health and require many disciplines to be answered."
Even in this short time, there are already some interesting achievements, says Braakman. "We recently identified an unexpected motif in CFTR that is essential for its folding process. A new postdoc has already improved an assay for kinetic analysis. I am very happy to see these results so quickly. It tells me that all lights are green for FLOW."
The grant is awarded for ten years, with the second half granted after evaluation. Braakman says: "In five years we should have the contours of the path from cradle to grave ready for both proteins. We should know what the important interactors are and whether they are unique or redundant. We should be able to reconstitute the QC triage process for folding versus aggregation or degradation in vitro. We would be extremely successful if we can execute refolding of CFTR in membrane-like structures."
"In ten years we aspire to be able to push a button and change the fate of a protein, for example by inhibiting a specific co-chaperone. FLOW is a very fundamental program, aimed at filling in the blanks in our knowledge, but this eventually can lead to new therapies."
SIDEBAR
Ubiquitin's role in the cell's quality control system
Monique Mulder is one of the principal investigators in the FLOW consortium. Her group at the Chemical Biology and Drug Discovery lab at LUMC specializes in understanding and modulating the role of the ubiquitin proteasome system in diseases, such as Huntington, a neurodegenerative disease caused by protein aggregation. "The ubiquitin proteasome system in the cell is one of the quality control systems. It controls the fate and degradation of proteins by ubiquitination. It therefore fits within the scope of the FLOW program perfectly," Mulder explains.
Mulder and her group use chemistry to understand ubiquitination processes by developing for example activity based probes, assay reagents and small molecules. So far, they have focussed on the ubiquitin enzymes involved in Huntington's disease, but within the FLOW consortium Mulder happily takes on the challenge to switch to alpha-synuclein (aSyn). "It is good to have a focus on the model proteins. The project interfaces with our research, but the protein is new to me. FLOW is a very interesting program as it brings together basic scientists from diverse fields. Many of the PI's are specialised in chaperones, a different but related part of the QC system. It is exciting that we will be challenged to think and work outside our own box."
The first PhD student started on November 1st. First, they will focus on synthesizing aSyn using peptide chemistry. This will allow them to do post-translational modifications of aSyn, including ubiquitination. "Many PTMs are known for aSyn, but we don't have a good understanding of their function. We will investigate the interactions and study which of more than 600 known E3 ligases are relevant for aSyn. We will also develop PROTACS which play a role in degradation of unwanted proteins. The overall goal is to be able to prevent aggregation."