Florian Winau, an associate professor of Pediatrics at Harvard Medical School (US) studies "basic mechanisms that explain how the immune system functions". As can be read on the groups’ website, they have a strong interest in identifying targets for possible translation to future immunotherapies. But if therapy is not an option, vaccination is a strategy to control infectious disease.
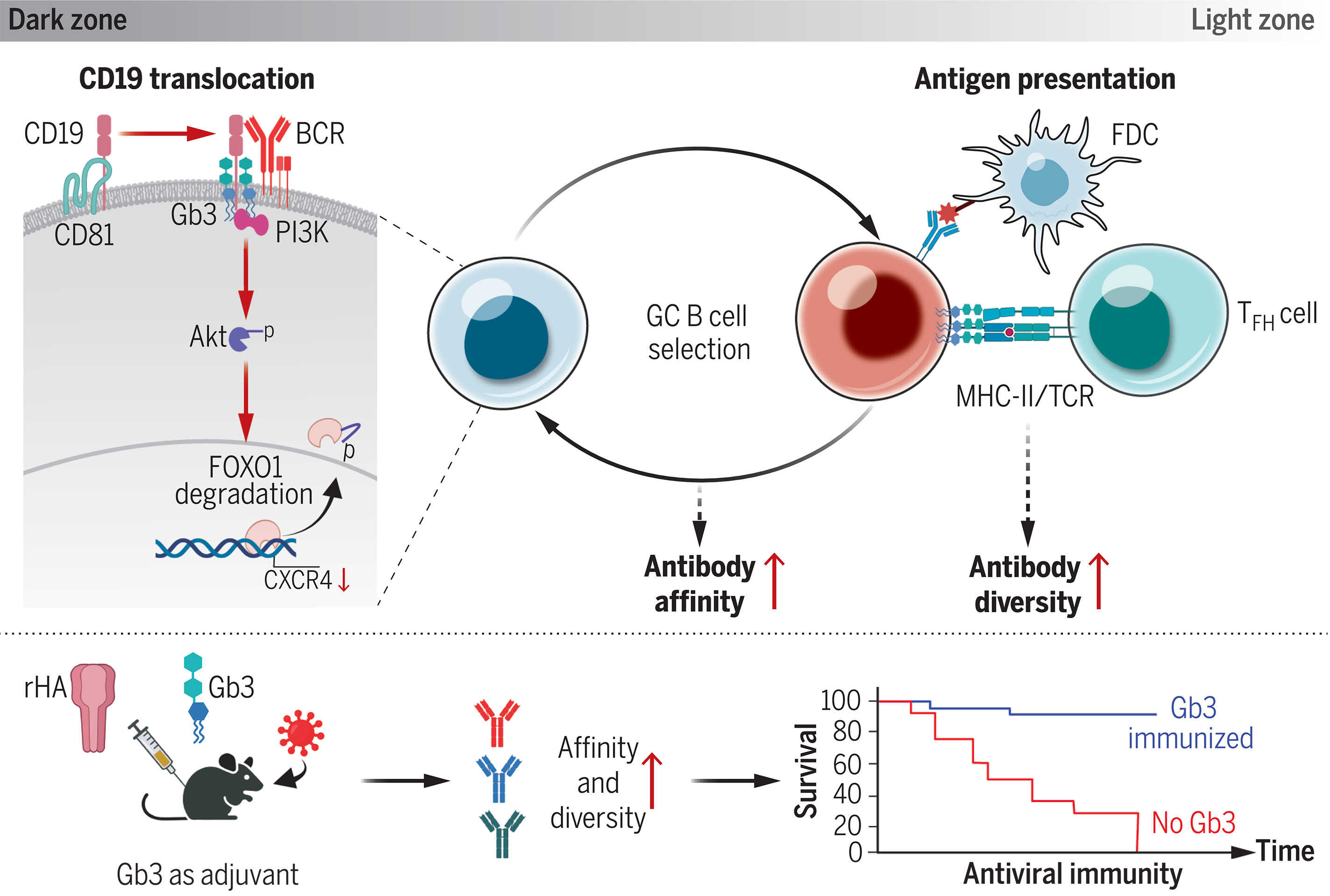
Vaccines induce the production of antibodies. These should not only have high affinity and broad reactivity but also be diverse to ensure their protective action when viral strains mutate. B lymphocytes that function in the humoral immunity component of the adaptive immune system, are recruited to the so-called germinal centers (GCs) where they mutate their antibody sequences and in this way promote diversity and antibody affinity maturation. A type of glycosphingolipid called globotriaosylceramide (Gb3) is abundantly found on the surface of these cells, but the researchers noted that it was unknown what role they play in the function of the B cells.
Mouse models
The researchers used genetically modified mouse models to investigate several steps in the process of B cell maturation and mutation (somatic hypermutation, B cell receptor signaling, GC B cell cycling, and MHC-II presentation) to understand the impact of the lipid Gb3 on antibody affinity and diversity.
They found that in a first step, Gb3 binds to the plasma membrane glycoprotein CD19. Binding detaches it from its chaperone CD81 allowing it to help bring the B cell to its receptor. Additionally, the researchers found that without Gb3 several other steps in the cascade could not take place such as FOXO1 degradation. This ultimately leads the researchers to conclude that "without Gb3, B cells were not able to undergo affinity maturation in germinal centers and failed to produce high-affinity antibodies." Additionally, they found that Gb3 amplifies antibody diversity by exposing subdominant epitopes that are not normally recognized in the immune response.
René Toes, immunologist at LUMC and not involved in this research, noted another interesting result in the publication: "Gb3 also increases type I interferon signalling. Although it is not described how this works, interferon signalling improves B cell response and therefore antibody response. This is relevant because interferon signalling is important in many other (auto)immune diseases such as lupus."
Finally, the researchers hypothesized that Gb3 could enhance the efficacy of influenza vaccines. They administred Gb3 to knockout mice and investigated the uptake of Gb3. They found that Gb3 was incorporated in the membrane of B cells and observed that this "promoted broadly reactive antibody responses and cross-protection" concluding that Gb3 could be an adjuvant in influenza vaccines.
Fundamental and applied
Toes is impressed by the complexity of the research combining chemistry and immunology, and the unexpected result. "They shed a fundamentally new light on the regulation of B cell response where ceramides play a role that was unexpected to me. The combination with the first application in a vaccine which showed the incorporation of Gb3 into the B cell membrane is remarkable. There is a lot of work ahead to apply this to human vaccines, but it is a good first step."